Vaccin Dos 3
8 mL of diluent 03 mL Additional Primary Dose for Moderately and Severely Immunocompromised Persons If administering Recipients age Use Mix Vaccine Using Administer Any dose in the series include the primary 2-dose series and an. Du kan få din första andra eller din påfyllnadsdos här.
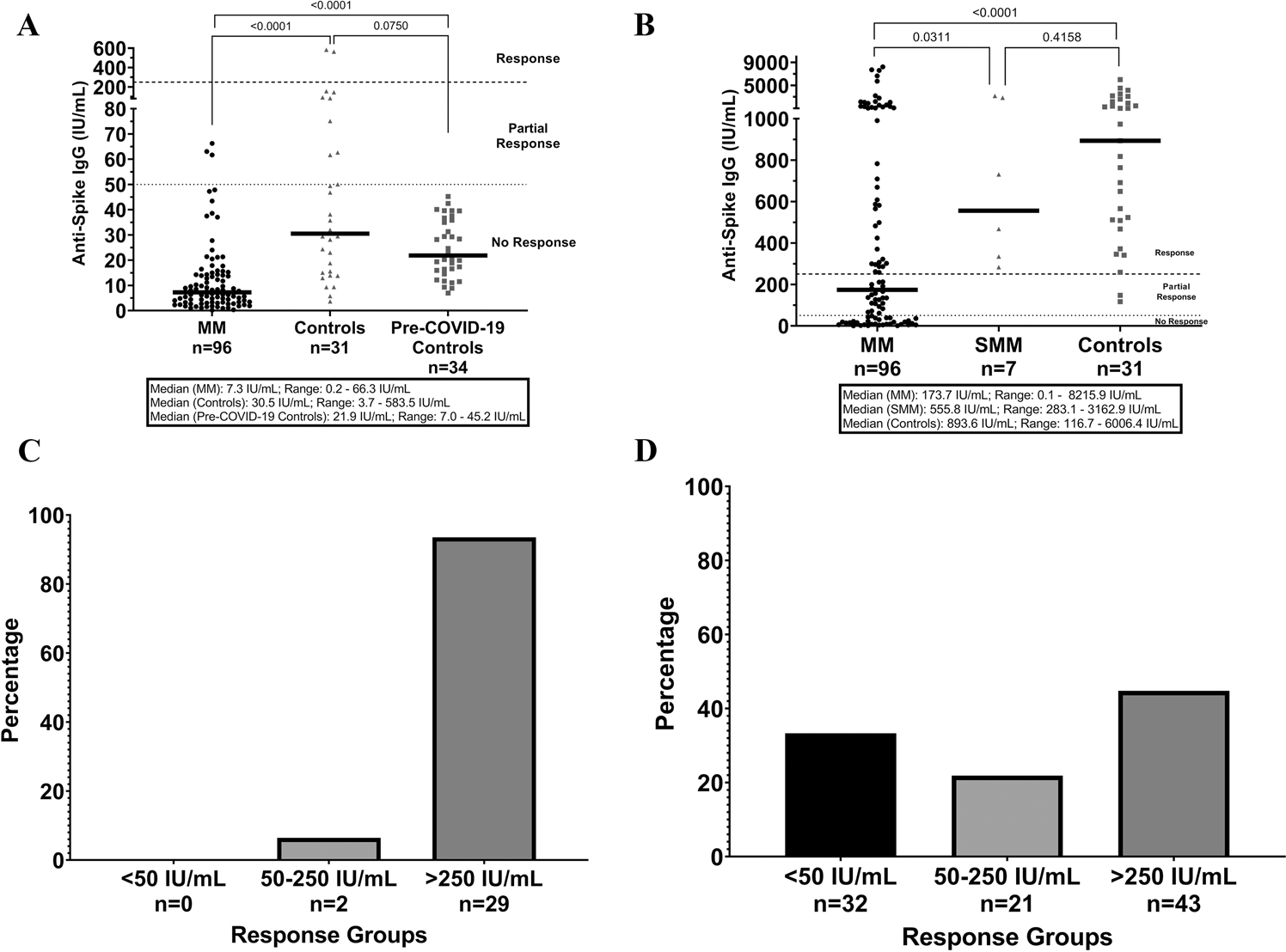
Response To Mrna Vaccination For Covid 19 Among Patients With Multiple Myeloma Leukemia
Folkhälsomyndigheten har idag beslutat att rekommendera att personer födda 1956 eller tidigare erbjuds en tredje dos vaccin mot covid-19.

. Are recommended to receive the first dose of hepatitis B vaccine within 24 hours of birth. Från och med måndag kan dos 3 bokas in på våra. Pfizer CEO Albert Bourla said people will likely need a third dose of a Covid-19 vaccine within 12 months of getting fully vaccinated.
On Friday the FDA cut the waiting period between the second dose of Modernas shot and a booster from sixth months down to five after making. Being properly hydrated will not only prevent you from feeling sick but may also help to shorten the duration and intensity of side effects. A booster shot is now approved for anyone ages 12 and up.
You should not get the second dose early. Given 6 months after additional primary shot. En fjärde dos vaccin mot covid-19 erbjuds dig som har ett kraftigt nedsatt immunförsvar på grund av sjukdom eller behandling och är.
According to CDC third shots of coronavirus vaccines significantly reduced the risk of hospitalization due to COVID in people with weakened immune systems - The New York Times. People age 18 can get a booster shot of any of the COVID-19 vaccines authorized in the United States. Some urge COVID-19 vaccine certificates to require 3 doses as Ontario set for reopening By Allison Jones The Canadian Press Posted January 30 2022 937 am.
Bokningen i Region Stockholm är redan öppen. He also said its possible people will need to get. Med en tredje dos vaccin förstärks och förlängs skyddet mot covid-19.
People age 12 who are moderately or severely immunocompromised should get an additional primary shot of Pfizer-BioNTech COVID-19 vaccine. COVID-19 Vaccine Third Dose Recommendations. On October 29 2021 the Food and Drug Administration FDA amended the Emergency Use Authorization EUA for Pfizer-BioNTech COVID-19 BNT162b2 mRNA vaccine to expand its use to children aged 511 years administered as 2 doses 10 μg 02mL each 3 weeks apart 1As of December 19 2021 only the Pfizer-BioNTech COVID-19 vaccine is authorized for.
This includes people who received another COVID-19 vaccine brand AstraZeneca for their first 2 doses. Personer födda 1941 eller tidigare samt personer med hemtjänst eller hemsjukvård är välkomna att boka in sig för en tredje dos vaccin sex månader efter dos 2. The most common side effects of vaccines include muscle pain fatigue headache and fever.
Vuxendosen används från 12 år och uppåt och rekommenderat intervall är 3-7 veckor. Given 28 days after 2 nd shot. 88 reduced risk two weeks after the third shot.
Severely immunocompromised individuals who have received 3 doses are recommended a booster dose fourth dose 4 months after their third dose. 3 You should get your second shot as close to the recommended 3-week or 4-week interval as possible. Nu påbörjas vaccination med en tredje dos på länets särskilda boenden.
4 As with vaccines for other diseases people who are up to date on their COVID-19 vaccines are optimally protected. Spikevax är ett mRNA-vaccin som är godkänt från 12 år. Antalet doser kan vara begränsade och ibland kan det.
On Wednesday Israel approved fourth doses of. Dos 3 för dig som är född 1956 eller tidigare. Till drop-in kommer du som inte vill boka tid för din vaccination.
Booster vaccinations are currently not recommended if you are aged under 18 years of age. 3 months 84 days after completion of the primary series page 5 Fourth doses for specific populations. 13 mL of diluent 02 mL 12 years of age and older 12 years of age and older formulation purple cap 1.
Highlights of changes Immunocompromised individuals who are eligible for a three-dose primary series may receive a booster dose. A third dose of the Pfizer-BioNTech vaccine is now approved for moderately or severely immunocompromised people ages 5 and up. And in the two-week period in mid-to-late August ER visits were 34 times higher in the states with the lowest vaccination rates while hospitalizations were 37 times higher than in states with.
In Israel on the other hand unless you received your second dose of the Covid-19 vaccine within the last six months you will now need a third dose to become eligible for a green pass which. Version 70 January 13 2022. The CDC updated its guidance this month to indicate that immunocompromised people can receive a fourth COVID-19 shot.
3-Dose Vaccine Series for Infants Including the Birth Dose Since 1991 ALL medically stable infants with a birth weight of at least 2000 g in the US. Staying hydrated is extremely important both before and after your vaccination. Om vaccin mot covid-19 Drop-in-vaccination mot covid-19 i Stockholms län Innehållet gäller Stockholms län.
Man behöver inte börja om eller ge någon extra dos om det går längre tid än sju veckor mellan den första och den andra dosen. We conducted a global randomized placebo-controlled phase 123 pivotal trial in which two 30-μg doses of BNT162b2 PfizerBioNTech were administered 21 days apart ClinicalTrialsgov number NCT04368728These doses of vaccine had mainly low-grade side effects and provided 95 efficacy against coronavirus disease 2019 Covid-19 from 7 days to. Om vaccin mot covid-19 Fjärde dos av vaccin mot covid-19 för dig med allvarligt nedsatt immunförsvar i Sörmland Innehållet gäller Sörmland.
For symptomatic disease all vaccine brands combined. Those who have recovered from Covid-19 are advised to take the vaccine booster dose three months after recovery Hamad General Hospital medical director Dr. Learn more about staying up to date on your COVID-19 vaccines.
Fler grupper kan nu få en tredje dos. Three doses of. The recommended wait time for a booster after receiving the Pfizer-BioNTech or Moderna vaccines has decreased from 6 months to 5 months.
This 3rd dose is recommended for all moderately-severely immunocompromised people ages 12 and older given 28 days after receipt of the second dose of their primary mRNA vaccine series and considered part of a 3-dose primary series for this select group of highly vulnerable people. Drink a lot of water.
What Happens If I Take A While To Get My Second Covid Vaccine Shot Tecnologico De Monterrey

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet
Covid 19 Dos And Don Ts After Vaccination Unicef India
Nu Planeras For Vaccinering Med Dos 3 Startsida

Vaccination Information Central Washington University

Dos And Donts During Covid Vaccination Vikaspedia

Human Papillomavirus Vaccination Uptake In Low And Middle Income Countries A Meta Analysis Eclinicalmedicine

Dos 3 Gor Skillnad Hog Tid Att Boka Vaccinationstid Region Dalarna
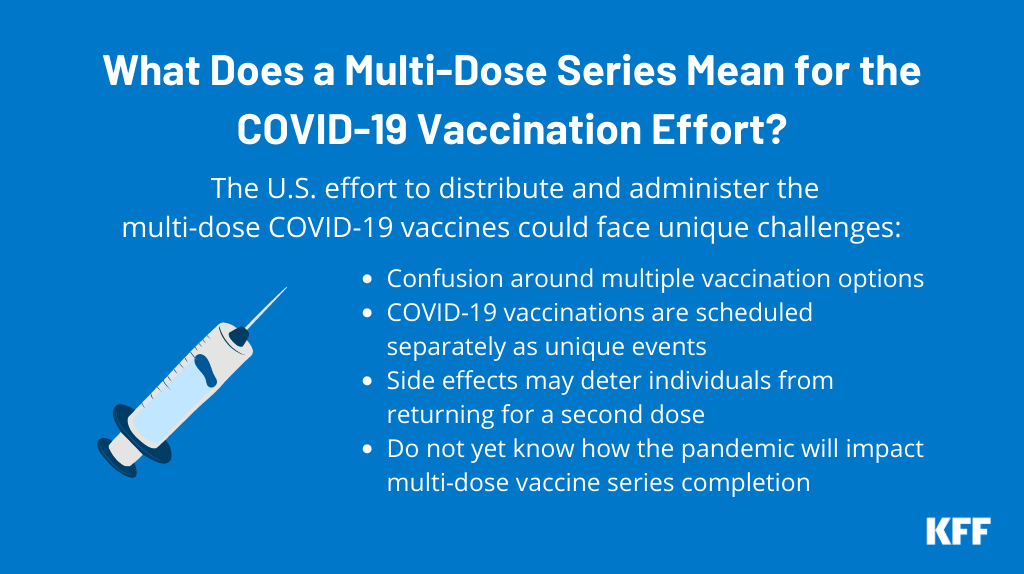
What Does A Multi Dose Series Mean For The Covid 19 Vaccination Effort Kff

Vi Borjar Vaccinera Med Dos 3 Karlsborgs Kommun

University Announces Compliance Processes For Federal Contractor Vaccine Mandate Penn State University
How To Keep The Vaccination Card With You Coronavirus Updates Npr

Vaccinering Med Dos 3 Mot Covid 19 Paborjas Valkommen Till Vaggeryds Kommuns Officiella Webbplats Har Hittar Du Information Och Nyheter Om Och Fran Kommunen